

The U.S. Access Board is a federal agency that promotes equality for people with disabilities through leadership in accessible design and the development of accessibility guidelines and standards for the built environment, transportation, communication, medical diagnostic equipment, and information technology.
Access Board logo Access Currents
News from the U.S. Access Board • January/ February 2020
- Board Initiates Update of Accessibility Guidelines for Rail Cars
- Study Panel Meets on Assessing the Feasibility of Equipping Passenger lanes with Wheelchair Restraint Systems
- Board to Hold Town Hall Meeting and Trainings in Philadelphia on May 6
- Board Executive Director David Capozzi to Retire
- Upcoming Board Webinars
- Board to Hold Next Meeting on March 11
- HUD Proposes to Adopt Additional Safe Harbors under the Fair Housing Act
- GAO Issues Report on Lack of Access to Aircraft Lavatories
- DOT Proposes New Rules on Aircraft Accessibility and Service Animals
Board Initiates Update of Accessibility Guidelines for Rail Cars
 The Access Board is initiating rulemaking to update its accessibility guidelines for rail cars covered by the Americans with Disabilities Act (ADA) and seeks public comment on this effort. As indicated in a recent Advance Notice of Proposed Rulemaking, the Board plans to update provisions in the ADA Accessibility Guidelines for Transportation Vehicles that apply to vehicles used in fixed guideway systems, including rapid, light, commuter, and intercity rail. The Board requests information from the public for its use in developing a proposed rule.
The Access Board is initiating rulemaking to update its accessibility guidelines for rail cars covered by the Americans with Disabilities Act (ADA) and seeks public comment on this effort. As indicated in a recent Advance Notice of Proposed Rulemaking, the Board plans to update provisions in the ADA Accessibility Guidelines for Transportation Vehicles that apply to vehicles used in fixed guideway systems, including rapid, light, commuter, and intercity rail. The Board requests information from the public for its use in developing a proposed rule.
The Board intends to update these guidelines, which were published in 1991, according to an advisory panel it organized. The Rail Vehicles Access Advisory Committee, which included representatives from advocacy organizations, transit operators, rail car manufacturers, and other stakeholders, reviewed the existing guidelines for rail vehicles and recommended how they should be updated to address accessibility issues, advances in technology, changes in car design, and other factors. The Committee’s report provides recommendations on provisions for vehicle communications, boarding and alighting, on-board circulation, seating, and rooms and spaces.
The advance notice discusses the committee’s report and includes questions posed by the Board. The Board seeks comment on both the substance of the recommendations from the committee as well as related questions about the feasibility or potential impacts on vehicle design, operations, and cost. It is also interested in research, data, and technologies on improved accessibility to rail vehicles.
Questions raised in the notice address coverage of new and remanufactured vehicles, variable message signs, hearing induction loops, vehicle ramps and lifts, car doors, between-car barriers, handrails and stanchions, wheelchair spaces, and vertical access in bi-level cars. The Board will use the information collected to draft a proposed rule which also will be made available for public comment.
The advance notice, which includes Instructions for submitting comments, is posted at regulations.gov (Docket ATBCB-2020-0002). Comments are due May 14, 2020. In addition, the Board will hold a public hearing on March 10 from 2:00 – 4:00 (ET) that will provide an opportunity to submit comments either in person or by phone. Those who wish to provide testimony should contact Rose Marie Bunales at (202) 272-0006![Call: (202) 272-0006]() or [email protected] .
or [email protected] .
For further information, visit the Board’s website or contact Juliet Shoultz at (202) 272-0045![Call: (202) 272-0045]() or [email protected] for technical questions or Wendy Marshall at (202) 272-0043
or [email protected] for technical questions or Wendy Marshall at (202) 272-0043![Call: (202) 272-0043]() or [email protected] for legal questions.
or [email protected] for legal questions.
Public Hearing on the ANPRM
March 10, 2020 2:00 – 4:00 (ET) Hearing Notice
Access Board Conference Center
1331 F Street, NW, Suite 800
Washington, D.C.
Contact: Rose Marie Bunales, (202) 272-0006![Call: (202) 272-0006]() , [email protected]
, [email protected]
Note: For the comfort of all participants and to promote a fragrance-free environment, attendees are requested not to use perfume, cologne, or other fragrances.
back to top
Study Panel Meets on Assessing the Feasibility of Equipping Passenger Planes with Wheelchair Restraint Systems
 The Access Board is assessing the feasibility of equipping passenger planes with wheelchair restraint systems through an expert panel organized by the Transportation Research Board (TRB). The Committee on the Feasibility of Wheelchair Restraint Systems in Passenger Aircraft held its first meeting February 5 and 6 at the Board’s conference space. At the meeting, the Board provided further direction to the committee on the scope of this project which will evaluate the design, engineering, and safety requirements for equipping aircraft with locking or tiedown mechanisms that would allow passengers to remain in their wheelchairs on flights. Members also reviewed the procedures that will govern its work and meetings.
The Access Board is assessing the feasibility of equipping passenger planes with wheelchair restraint systems through an expert panel organized by the Transportation Research Board (TRB). The Committee on the Feasibility of Wheelchair Restraint Systems in Passenger Aircraft held its first meeting February 5 and 6 at the Board’s conference space. At the meeting, the Board provided further direction to the committee on the scope of this project which will evaluate the design, engineering, and safety requirements for equipping aircraft with locking or tiedown mechanisms that would allow passengers to remain in their wheelchairs on flights. Members also reviewed the procedures that will govern its work and meetings.
The agenda featured presentations by invited quests and stakeholders. Representatives from organizations that have provided leadership, advocacy, and research on this subject, including All Wheels Up, Flying Disabled, and Paralyzed Veterans of America, presented information that has been collected to date. An official from the Federal Aviation Administration briefed members on relevant regulatory requirements and other considerations. Speakers from Airlines for America, the National Air Carrier Association, and Boeing also briefed the committee. The meeting also provided opportunities for members of the public to raise comments or questions.
The committee’s 13 members include experts in aircraft interiors and safety engineering, accessibility, wheelchair design and crashworthiness, airline operations, and other disciplines. If the committee finds that wheelchair restraint systems on aircraft are feasible, it will proceed to assess how passengers using wheelchairs can be accommodated through all phases of flight, from boarding to deplaning. The committee’s next meeting will be held April 20 and 21. Most sessions are open to the public and are webcast.
Further information on this study is available on TRB’s website. Questions about the project can be directed to Mario Damiani of the Access Board at [email protected] or (202) 272-0050![Call: (202) 272-0050]() or Anusha Jayasinghe of TRB at [email protected] or (202) 334-2401
or Anusha Jayasinghe of TRB at [email protected] or (202) 334-2401![Call: (202) 334-2401]() .
.
back to top
Board to Hold Town Hall Meeting and Trainings in Philadelphia on May 6
 On May 6 the Board will conduct a town hall meeting in Philadelphia at Temple University Center City. The event, which will take place from 2:00 to 5:00 p.m., will provide an opportunity for members of the public to pose questions to the Board or to share comments or concerns about accessibility for people with disabilities. There also will be presentations by guest speakers on several different topics on accessibility and accessible design.
On May 6 the Board will conduct a town hall meeting in Philadelphia at Temple University Center City. The event, which will take place from 2:00 to 5:00 p.m., will provide an opportunity for members of the public to pose questions to the Board or to share comments or concerns about accessibility for people with disabilities. There also will be presentations by guest speakers on several different topics on accessibility and accessible design.
The Board will also conduct free training sessions earlier that day. One program will address the ADA Accessibility Standards and common sources of confusion (9:00 – 10:30). This will be followed by a session on accessible public rights-of-way (11:00 – 12:30). Both sessions are open to the public, and registration is not required. The Board will issue further details on the trainings and the town hall forum and post them on its website.
back to top
Board Executive Director David Capozzi to Retire
 At the January meeting of the Board, Executive Director David Capozzi announced plans to retire this spring. Capozzi joined the Board in 1992 as Director of the Office of Technical and Information Services. In 2008, he became Executive Director.
At the January meeting of the Board, Executive Director David Capozzi announced plans to retire this spring. Capozzi joined the Board in 1992 as Director of the Office of Technical and Information Services. In 2008, he became Executive Director.
During his 28-year tenure, the Board’s mission expanded significantly, and he led the development of new accessibility guidelines and standards in a variety of areas. This work included updating the Board’s guidelines for facilities and vehicles and issuing new standards for information and communication technology (ICT) in the federal sector and for medical diagnostic equipment (MDE). He oversaw rulemaking that broke new ground by addressing accessibility in areas for the first time, including not only the ICT and MDE standards, but also final guidelines for judicial and correctional facilities, outdoor developed areas, swimming pools, play areas, golf courses, and other recreation facilities, as well as proposed guidelines for public rights-of-way and passenger vessels.
“I’m proud of the work we accomplished during my time at the Board. We helped make the country more accessible,” he noted in announcing his decision to the Board. He expressed his admiration for the many colleagues at the Board, both current and former, he worked with over the years. “It’s like a family, and no one could ask for a better group of people to work with.”
Before joining the Board, Capozzi worked at Easter Seals as vice president of advocacy and director of Project ACTION. He also served as national advocacy director at the Paralyzed Veterans of America.
He plans to retire in late May or until his successor is named. The vacancy for the Executive Director position will soon be posted on the USAJobs website.
back to top
Upcoming Board Webinars
 Determining what is required to be accessible when a facility is altered or expanded can be a challenge. The next webinar in the Access Board’s free monthly series will take place March 5 from 2:30 – 4:00 (ET) and review how to apply the ADA and ABA Accessibility Standards to planned alterations and additions. Presenters will clarify common sources of confusion, including what type of work constitutes an “alteration,” how the scope of work determines application, the provisions for primary function areas and accessible paths of travel, historic facilities, and technical infeasibility.
Determining what is required to be accessible when a facility is altered or expanded can be a challenge. The next webinar in the Access Board’s free monthly series will take place March 5 from 2:30 – 4:00 (ET) and review how to apply the ADA and ABA Accessibility Standards to planned alterations and additions. Presenters will clarify common sources of confusion, including what type of work constitutes an “alteration,” how the scope of work determines application, the provisions for primary function areas and accessible paths of travel, historic facilities, and technical infeasibility.
For more information or to register, visit www.accessibilityonline.org. Questions can be submitted in advance of the session (total limited to 25) or can be posed during the live webinar. Webinar attendees can earn continuing education credits. The webinar series is hosted by the ADA National Network in cooperation with the Board. Archived copies of previous Board webinars are available on the site.
Section 508 Best Practices Webinar
The Board also offers a free webinar series on standards issued under Section 508 of the Rehabilitation Act which requires access to information and communication technology (ICT) in the federal sector. The next webinar in this series will take place on March 31 from 1:00 – 2:30 (ET) and cover how to provide access to social media. The presenters will provide an overview of social media techniques, address common questions, review access issues and solutions, and offer best practices and techniques for making content accessible on various social media platforms, including Facebook, Flickr, Google+, Twitter, and YouTube. Questions can be submitted in advance of the session or can be posed during the live webinar.
For more details or to register, visit www.accessibilityonline.org/cioc-508/schedule. The Section 508 Best Practices Webinar Series is made available by the Accessibility Community of Practice of the CIO Council in partnership with the Board.
back to top
Board to Hold Next Meeting on March 11
 The Access Board will hold its next meeting March 11 from 1:30 – 3:00 (ET) at the Board’s conference space in downtown Washington, D.C. The public is welcome to attend in person or through a live webcast of the meeting. The meeting agenda includes election of Board officers and other activities.
The Access Board will hold its next meeting March 11 from 1:30 – 3:00 (ET) at the Board’s conference space in downtown Washington, D.C. The public is welcome to attend in person or through a live webcast of the meeting. The meeting agenda includes election of Board officers and other activities.
A public comment period will be held during the final 15 minutes of the meeting. Those interested in making comments in person or by phone should send an email to Rose Bunales at [email protected] with “Access Board meeting – Public Comment” in the subject line. Please include your name, organization, state, and topic of your comment in the body of the message.
Meeting of the U.S. Access Board
March 11, 1:30 – 3:00 (ET)
Webcast link: www.access-board.gov/webcast
Access Board Conference Center
1331 F Street, NW, Suite 800
Washington, D.C.
Note: For the comfort of all participants and to promote a fragrance-free environment, attendees are requested not to use perfume, cologne, or other fragrances.
back to top
HUD Proposes to Adopt Additional Safe Harbors under the Fair Housing Act
 The Department of Housing and Urban Development (HUD) has issued a notice to adopt additional safe harbor standards under the Fair Housing Act (FHA). The FHA bans discrimination in housing on the basis of disability, race, color, gender, and religion. To ensure compliance with accessibility requirements, HUD implemented the Fair Housing Accessibility Guidelines which apply to the design and construction of multi-family housing of four or more units. The FHA regulations recognize several alternatives to these guidelines as “safe harbors” under the Act. These include early editions of the International Building Code (IBC), which contains requirements for accessible housing that correspond to the FHA requirements, and the A117.1 Standard for Accessible and Usable Buildings and Facilities which includes technical provisions for accessible dwelling units.
The Department of Housing and Urban Development (HUD) has issued a notice to adopt additional safe harbor standards under the Fair Housing Act (FHA). The FHA bans discrimination in housing on the basis of disability, race, color, gender, and religion. To ensure compliance with accessibility requirements, HUD implemented the Fair Housing Accessibility Guidelines which apply to the design and construction of multi-family housing of four or more units. The FHA regulations recognize several alternatives to these guidelines as “safe harbors” under the Act. These include early editions of the International Building Code (IBC), which contains requirements for accessible housing that correspond to the FHA requirements, and the A117.1 Standard for Accessible and Usable Buildings and Facilities which includes technical provisions for accessible dwelling units.
HUD’s regulations currently designate as safe harbors housing requirements in early editions of the IBC (2000 with 2001 Supplement, 2003, and 2006) and those of the A117.1 standards (1986, 1992, 1998, and 2003). HUD’s recent notice proposes to also adopt as FHA safe harbors later editions of the IBC and A117.1 standard, which is what most states currently reference. These include the 2009 edition of the A117.1 standard and each of the succeeding editions of the IBC (2009, 2012, 2015, and 2018). As indicated in its notice, HUD reviewed each of these versions of the IBC and A117.1 standard and determined that they are no less stringent than the FHA guidelines or other designated safe harbors.
The notice is available on regulations.gov and open for public comment until March 16, 2020. For further information, visit HUD’s website or contact Lynn Grosso, Director of Enforcement at HUD’s Office of Fair Housing and Equal Opportunity, at (202) 708-2333![Call: (202) 708-2333]() or [email protected].
or [email protected].
back to top
GAO Issues Report on Lack of Access to Aircraft Lavatories
 The Government Accountability Office (GAO) recently completed a study on the accessibility of lavatories on single-aisle aircraft. Under current federal regulations, accessible lavatories are required on twin-aisle aircraft but not single-aisle aircraft. However, single-aisle planes are increasingly being used on longer flights.
The Government Accountability Office (GAO) recently completed a study on the accessibility of lavatories on single-aisle aircraft. Under current federal regulations, accessible lavatories are required on twin-aisle aircraft but not single-aisle aircraft. However, single-aisle planes are increasingly being used on longer flights.
Congress directed GAO to undertake the study which examined lavatory design options offered by aircraft manufacturers and challenges passengers with mobility impairments face accessing lavatories on single-aisle aircraft. GAO also collected information from the Department of Transportation (DOT), including complaint data, and consulted aircraft manufacturers, air carriers, disability organizations and consumer groups, and cabin crew associations.
GAO reviewed available designs for lavatories that can accommodate passengers using onboard wheelchairs. One design features a larger lavatory for installation on single-aisle aircraft. Another consists of two adjacent lavatories that can be combined into one when needed for accessibility. According to GAO, aircraft manufacturers offer these designs, but air carriers often do not choose to acquire them. GAO collected data on the fleets of the eight largest U.S. carriers and found that among the total number of single-aisle aircraft with 100 or more seats, only 4.5% (161 of 3,555) had lavatories designed for improved accessibility.
For further information on the findings of the study, visit GAO’s website or contact Andrew Von Ah at (202) 512-2834![Call: (202) 512-2834]() or [email protected]. In addition, DOT is undertaking rulemaking on this subject as well (next article).
or [email protected]. In addition, DOT is undertaking rulemaking on this subject as well (next article).
back to top
DOT Proposes New Rules on Aircraft Accessibility and Service Animals
 The Department of Transportation (DOT) has issued two proposed rules for public comment on air travel and accessibility for passenger with disabilities. In early January, it proposed new requirements designed to facilitate access to lavatories for passengers using onboard wheelchairs. These include provisions for assist handles, lower door sills, attendant call buttons, and controls and faucets that are easier to use. The rule would not increase the size of lavatories.
The Department of Transportation (DOT) has issued two proposed rules for public comment on air travel and accessibility for passenger with disabilities. In early January, it proposed new requirements designed to facilitate access to lavatories for passengers using onboard wheelchairs. These include provisions for assist handles, lower door sills, attendant call buttons, and controls and faucets that are easier to use. The rule would not increase the size of lavatories.
The proposed rule also includes performance standards for onboard wheelchairs which are used to provide access to aircraft lavatories. The Board is developing advisory technical guidelines for onboard wheelchairs, which it previously released for comment, that DOT’s rule may reference as a voluntary baseline for meeting the proposed performance criteria.
DOT’s rule also would require training of flight attendants on assisting passengers with disabilities, and the posting of information on the access features of a plane’s lavatory. The proposed rule is posted on regulations.gov. For further information, contact Robert Gorman of DOT’s Office of Aviation Enforcement and Proceedings at (202) 366-9342![Call: (202) 366-9342]() or [email protected].
or [email protected].
Proposed Rule on Service Animals on Aircraft
In a separate rulemaking, DOT has proposed changes to provisions in its Air Carrier Access Act (ACAA)regulations that address service animals in air travel. Under the proposed revisions, which are open for comment, only dogs individually trained as service animals would be recognized as service animals by ACAA regulations. Other animals, including emotional support animals, would not. In addition, airlines would be able to require passengers travelling with service animals to check in at least one hour earlier than the general boarding deadline. It also would allow airlines to require documentation of a service animal’s abilities and health.
The proposed rule is available for comment until April 6, 2020 on regulations.gov. For further information, visit DOT’s website or contact Maegan Johnson of DOT’s Office of Aviation Enforcement and Proceedings at (202) 366-9342![Call: (202) 366-9342]() or [email protected].
or [email protected].
Post Views: 1,281

 Nice writeup by Freedom Scientific on what a screen reader is.
Nice writeup by Freedom Scientific on what a screen reader is.




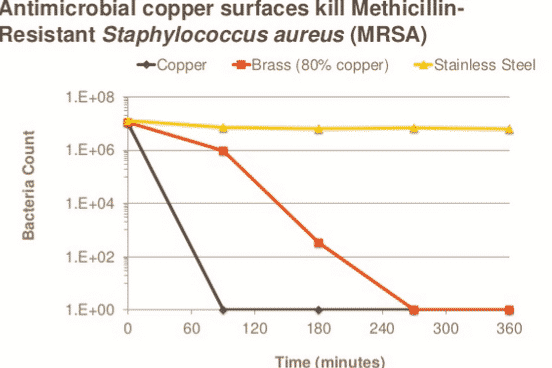
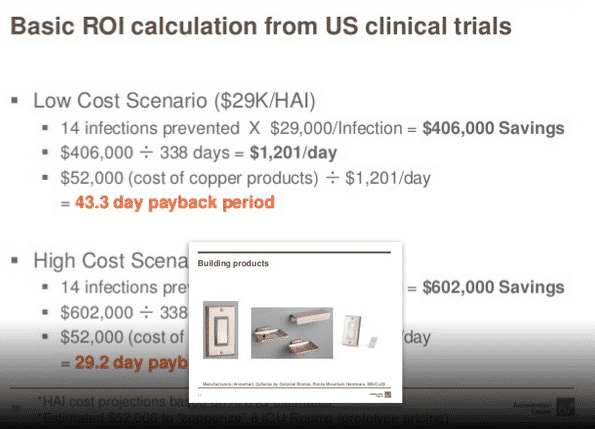

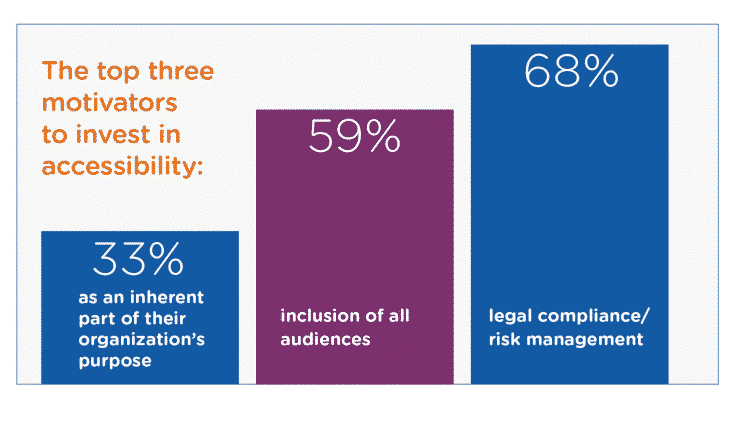 By Rob Sinclair –
By Rob Sinclair – 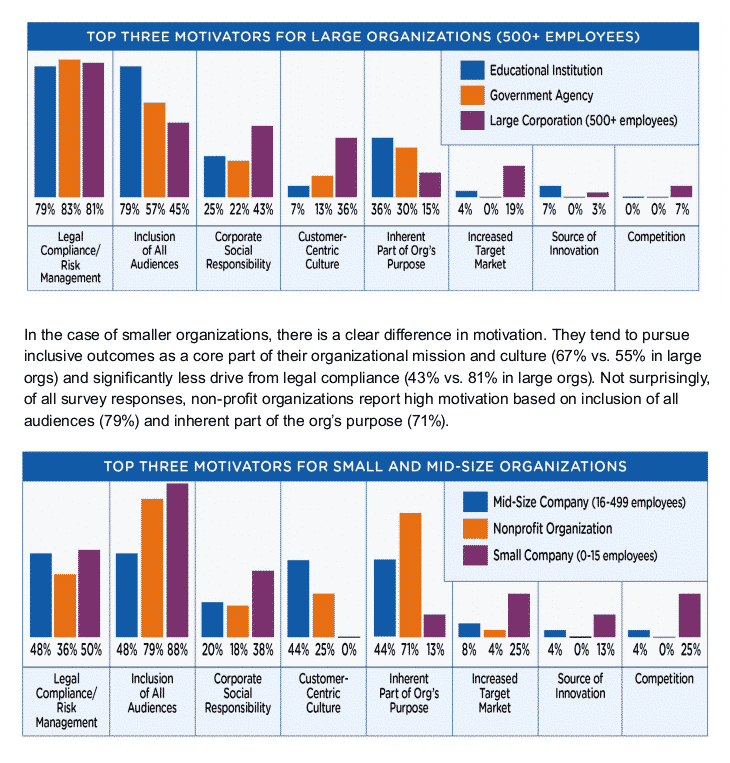

 Web Content Accessibility Guidelines (WCAG) 2.2 covers a wide range of recommendations for making Web content more accessible. Following these guidelines will make content more accessible to a wider range of people with disabilities, including accommodations for blindness and low vision, deafness and hearing loss, limited movement, speech disabilities, photosensitivity, and combinations of these, and some accommodation for learning disabilities and cognitive limitations; but will not address every user need for people with these disabilities. These guidelines address accessibility of web content on desktops, laptops, tablets, and mobile devices. Following these guidelines will also often make Web content more usable to users in general.
Web Content Accessibility Guidelines (WCAG) 2.2 covers a wide range of recommendations for making Web content more accessible. Following these guidelines will make content more accessible to a wider range of people with disabilities, including accommodations for blindness and low vision, deafness and hearing loss, limited movement, speech disabilities, photosensitivity, and combinations of these, and some accommodation for learning disabilities and cognitive limitations; but will not address every user need for people with these disabilities. These guidelines address accessibility of web content on desktops, laptops, tablets, and mobile devices. Following these guidelines will also often make Web content more usable to users in general.

 The
The  The Access Board is assessing the feasibility of equipping passenger planes with wheelchair restraint systems through an expert panel organized by the Transportation Research Board (TRB). The Committee on the Feasibility of Wheelchair Restraint Systems in Passenger Aircraft held its first meeting February 5 and 6 at the Board’s conference space. At the meeting, the Board provided further direction to the committee on the scope of this project which will evaluate the design, engineering, and safety requirements for equipping aircraft with locking or tiedown mechanisms that would allow passengers to remain in their wheelchairs on flights. Members also reviewed the procedures that will govern its work and meetings.
The Access Board is assessing the feasibility of equipping passenger planes with wheelchair restraint systems through an expert panel organized by the Transportation Research Board (TRB). The Committee on the Feasibility of Wheelchair Restraint Systems in Passenger Aircraft held its first meeting February 5 and 6 at the Board’s conference space. At the meeting, the Board provided further direction to the committee on the scope of this project which will evaluate the design, engineering, and safety requirements for equipping aircraft with locking or tiedown mechanisms that would allow passengers to remain in their wheelchairs on flights. Members also reviewed the procedures that will govern its work and meetings. On May 6 the Board will conduct a town hall meeting in Philadelphia at Temple University Center City. The event, which will take place from 2:00 to 5:00 p.m., will provide an opportunity for members of the public to pose questions to the Board or to share comments or concerns about
On May 6 the Board will conduct a town hall meeting in Philadelphia at Temple University Center City. The event, which will take place from 2:00 to 5:00 p.m., will provide an opportunity for members of the public to pose questions to the Board or to share comments or concerns about  At the January meeting of the Board, Executive Director David Capozzi announced plans to retire this spring. Capozzi joined the Board in 1992 as Director of the Office of Technical and Information Services. In 2008, he became Executive Director.
At the January meeting of the Board, Executive Director David Capozzi announced plans to retire this spring. Capozzi joined the Board in 1992 as Director of the Office of Technical and Information Services. In 2008, he became Executive Director. Determining what is required to be accessible when a facility is altered or expanded can be a challenge. The next
Determining what is required to be accessible when a facility is altered or expanded can be a challenge. The next  The Access Board will hold its next meeting March 11 from 1:30 – 3:00 (ET) at the Board’s conference space in downtown Washington, D.C. The public is welcome to attend in person or through a live webcast of the meeting. The meeting agenda includes election of Board officers and other activities.
The Access Board will hold its next meeting March 11 from 1:30 – 3:00 (ET) at the Board’s conference space in downtown Washington, D.C. The public is welcome to attend in person or through a live webcast of the meeting. The meeting agenda includes election of Board officers and other activities. The Department of Housing and Urban Development (HUD) has issued a notice to adopt additional safe harbor standards under the Fair Housing Act (FHA). The FHA bans discrimination in housing on the basis of disability, race, color, gender, and religion. To ensure compliance with accessibility requirements, HUD implemented the Fair Housing Accessibility Guidelines which apply to the design and construction of multi-family housing of four or more units. The FHA regulations recognize several alternatives to these guidelines as “safe harbors” under the Act. These include early editions of the International Building Code (IBC), which contains requirements for accessible housing that correspond to the FHA requirements, and the A117.1 Standard for Accessible and Usable Buildings and Facilities which includes technical provisions for accessible dwelling units.
The Department of Housing and Urban Development (HUD) has issued a notice to adopt additional safe harbor standards under the Fair Housing Act (FHA). The FHA bans discrimination in housing on the basis of disability, race, color, gender, and religion. To ensure compliance with accessibility requirements, HUD implemented the Fair Housing Accessibility Guidelines which apply to the design and construction of multi-family housing of four or more units. The FHA regulations recognize several alternatives to these guidelines as “safe harbors” under the Act. These include early editions of the International Building Code (IBC), which contains requirements for accessible housing that correspond to the FHA requirements, and the A117.1 Standard for Accessible and Usable Buildings and Facilities which includes technical provisions for accessible dwelling units. The Government Accountability Office (GAO) recently completed a study on the accessibility of lavatories on single-aisle aircraft. Under current federal regulations, accessible lavatories are required on twin-aisle aircraft but not single-aisle aircraft. However, single-aisle planes are increasingly being used on longer flights.
The Government Accountability Office (GAO) recently completed a study on the accessibility of lavatories on single-aisle aircraft. Under current federal regulations, accessible lavatories are required on twin-aisle aircraft but not single-aisle aircraft. However, single-aisle planes are increasingly being used on longer flights. The Department of Transportation (DOT) has issued two proposed rules for public comment on air travel and accessibility for
The Department of Transportation (DOT) has issued two proposed rules for public comment on air travel and accessibility for